NCL’s constant endeavors to research & most customer-specific requirements have resulted in the manufacture of a wide range of capsule shells. The cellulose capsule shells and newly introduced variants form a part of NCL’s niche products. NCL’s facility of dedicated manufacturing lines adopt stringent procedures to manufacture hard gelatin capsule shells. Each of these manufacturing lines is housed as an independent unit to eliminate the risk of cross contamination.
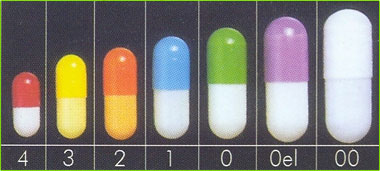
NCL manufactures empty capsule shells for pharmaceutical and dietary supplement industries in sizes 00, 0el, 0, 1, 2, 3 & 4. All operations are assured through our comprehensive QA procedures to conform to Indian Pharmacopoeia and United States Pharmacopoeia standards and the specific requirements of the customers.
• are easy to swallow
• mask odors
• are tasteless
• are aesthetically pleasing; and
• are versatile
• Can be filled in-house under a company’s direct supervision and control standards
• Require fewer excipients than other drug formulations
• Require less initial investment in processing equipment
• Are manufactured to universal standards, which allows for multi-sourcing and runnability on all types of filling equipment
• Offer endless varieties of color and print options for unique identification of your product in the market place
Recent survey results reveal that pharmaceutical customers choose capsule shell manufacturers from India based on the following characteristics:
• The Best Quality & Machinability
• Adherence to Timely Delivery
• Customer Service from Informative Staff
• Technology transfer from Knowledgeable Source
The gelatin capsule shell is the most common form of capsule for oral dosage formulations. NCL offers its high-quality products to customers committing to:
• Validated Manufacturing process based on cGMP’s
• Odorless & Tasteless Capsules
• Round the World Availability
• Five Year Shelf Life
• Preservative and Preservative-Free Capsules shells
• Made from pure Bovine Gelatin
A quality assurance system has been set up to be preventive and not curative. Our capsule shells undergo vigorous in-process inspection checks in accordance with cGMP and ISO 9001:2000. With every delivery, we provide our customers a bulletin of physico-chemical and bacteriological analyses in conformity with the requirements of Indian and United States Pharmacopoeia.